
Rakhmailov
Quantum-chemical hydro conversion of fuel and energy

«Since childhood everyone knows that, it is and it is impossible. But there’s one ‘ignoramus’ who doesn’t know it. He makes a discovery».
Albert EinsteinChemical energy has special significance in a modern society since chemical reactions are the major source of
Such a speed ratio is characteristic at explosions and detonation of
Triggering energy and fuel conversion is referred to
Three steps of established research of fuel and energy conversion.
- Step 1 — search for ways of increasing chemical energy conversion speed (CEC) in
ultra-lean mixtures (air excess factor of 4…5 units) with FAM containing: less than 5% of methane, less than 12% of oxygen, more than 30% of various CO2 combinations, more than 40% of H2O vapors and 0…85% of N2. Tests and optimization of technology were carried out at a specializedtest-bench . No known processing methods to increase CEC speed (increasing initial temperature and FAM pressure; implementing known brand new chemical reaction trigger sources) were applied. Research has been completed. Experiments showed positive outcome. Technology has been patented. USP No. 7,086,854. - Step 2 — developed technology of
high-speed CEC inultra-lean FAMs was adapted for chemical energy conversion contained in lean FAMs (air excess factor of 0.99…1.99 units). Research was carried out at specializedtest-bench and at operating 60 kW GTE. Research is finished. Experiments showed positive outcome. - Step 3 — investigated potential of developed technology to increase fuel chemical energy conversion to kinetic energy of conversion products. Research was carried out at specialized
test-bench and at operating 1500 kW GTE. In quantum reactor tested attest-bench less than 15% of fuel chemical energy is converted to kinetic energy. In quantum reactor -more than 30% of fuel chemical energy is converted to kinetic energy of conversion products. Research is still in progress. Technology is being patented.
Analysis of results had major
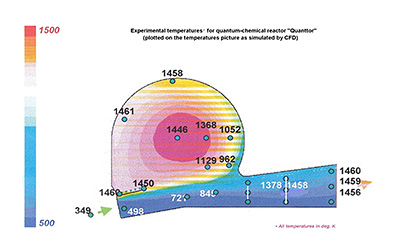
Picture 1: Experimental
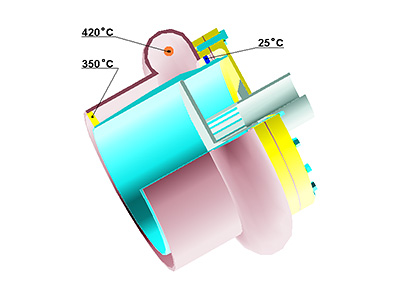
In the Quanttor reactor demonstrated at the picture -study of the processed managed energy and mass exchange between «cold» MAM (15…45 °C, methane content of 2.5… 10% abs.) and «hot» (350…1200 °C) conversion products at pressure rates exceeding atmosphere pressure (1…10 atmospheres).
Quanttor reactor provides the opportunity to perform managed hydro conversion of
A model to define critical conditions of
We have experimentally ascertained that chemical reactions involving converted fuel (methane):
- Proceed fast enough even at conversion temperature of 350 °C (Picture 2);
- 3–4 ms is sufficient time for chemical reactions inside the reactor (optimal conversion temperature — 1150…1200 °C) to be fully completed;
- Methane activation energy equals 5…6 kcal/mole;
- Fuel conversion process is not significantly dependent on temperature;
- No thermal gradient is observed within the conversion zone.
5% methane content in MAM is the level above, which a misbalance emerges in hydro conversion process between the energy required to convert fuel and the energy, produced while converting chemical energy. Further increase of methane concentration in MAM goes along with proportional decrease in temperature of conversion products output from the quantum reactor.
Due to involvement of
Picture 2: Minimal methane conversion temperature (experiment).
To create a revolutionary, highly economical, and exceptionally clean
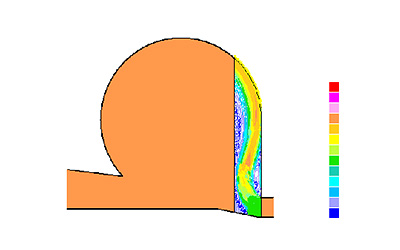
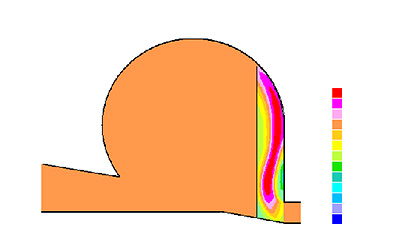
Picture 3: Temperature profile inside Quanttor reactor.
Picture 4: Velocity diagram inside
Picture 5: Radial velocity at entrance to
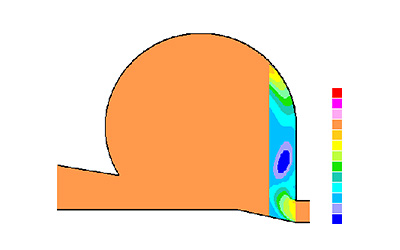
Picture 6: Axis velocity at entrance to
One should also note the phenomenal ability of Quanttor quantum reactor to run on mixtures containing
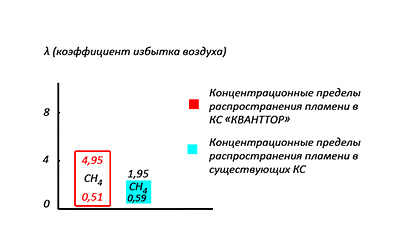
Picture 7: Concentration limits of flame propagation inside CC and
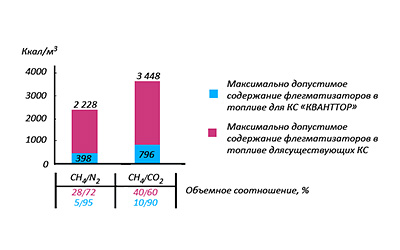
Picture 8: Composition and calorific capacity of
Hydrocarbon fuel hydro conversion Quanttor technology being patented was tested at the
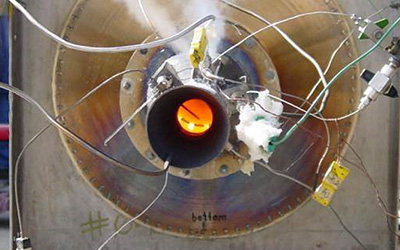
Picture 9: Quanttor reactor at the

Picture 10: Test-rig of Alturdyne Company where it was confirmed that the Quanttor reactor could run as part of GTE. On the left: A. M. Rakhmailov, Author of the technology.
Quanttor reactor snapshot:
- 1. Energy conversion completeness, %
- 100
- 2. NOx content in conversion products (15% of O2), ppm under
- 2
- 3. The minimum quantity of methane in MAM (LBO), %
- 2,5
- 4. Conversion temperature, °C
- min.
- 320
- optimal
- 1200
- 5. Optimal methane content in MAM, %
- 4,25
- 6. Optimal oxygen content in MAM, %
- 10,5
- 7. Max. acceptable CO2 content in fuel, %
- 35
- 8. Max. acceptable N2 content in fuel, %
- 45
Conclusions:
- Quantum-chemical Quanttor reactor is an appliance for hydro conversion of fuel (obtaining hydrogen and CO) and energy (converting energy from chemical reaction directly to kinetic energy of products resulting from the specified chemical reactions).
- Quanttor quantum-chemical reactor utilizes chemical processes with high speed exceeding the speed of equilibrium distribution of energy released during conversion.
